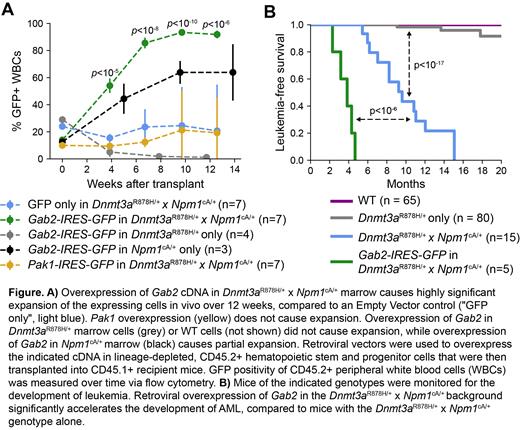
NPM1 and DNMT3A are among the most commonly mutated genes in Acute Myeloid Leukemia (AML), affecting 25-30% of patients. Importantly, these mutations co-occur much more frequently than expected by chance, with ~15% of AML samples containing mutations in both genes.This synergy is recapitulated in mouse models, where mice with either Dnmt3aR878H or Npm1cA mutations develop leukemia with a long latency and low penetrance, whereas mice with both Dnmt3aR878H and Npm1cA mutations develop leukemia in 8-12 months with nearly 100% penetrance (Dovey et al., Blood, 2017; Loberg et al., Leukemia, 2019).To better understand the mechanisms underlying this synergy, and the process of leukemic transformation, we have characterized pre-leukemic bone marrow and 9 independent spontaneous AMLs that have arisen in these mice. These spontaneous AMLs rapidly cause fatal leukemias in secondary recipients; whole genome sequencing of all tumors revealed one or more human AML-like cooperating mutations in each, including canonical mutations in Ptpn11 (E69K, A72V and S506L), an activating KitD818Y mutation, a Cbl exon 9 deletion, a CblY369H mutation,and an amplification of Flt3 associated with >20 fold overexpression of WT Flt3. Characterization of the pre-leukemic transcriptomes and methylomes demonstrated little change in the pre-leukemic state for several months, followed by dramatic and canonical shifts in transcription and methylation after transformation. Remarkably, 8 of the 9 AMLs contained an amplification of the entirety of murine chromosome 7 as the sole structural variant in each genome. We analyzed panel sequencing data from a set of published AMLs arising in this model (Loberg et al., Leukemia, 2019), and also exome sequencing data from a different Npm1 cA driven tumor model (Dovey et al., Blood, 2017); these AMLs also developed complete or partial amplification of chromosome 7 in 7 of 8 and 10 of 15 cases, respectively. Through integration of these datasets, we identified a minimally amplified 8.9 Mbp region on chromosome 7, containing 209 genes, including two candidate genes ( Gab2, Pak1) that show overexpression in both murine and human AMLs with DNMT3A and NPM1 mutations. Retroviral constructs expressing each cDNA (with an IRES-GFP tag) were transduced into lineage depleted bone marrow cells from preleukemic WT, Dnmt3aR878H, Npm1cA or Dnmt3aR878H x Npm1cA mice, and transplanted into recipient mice. Overexpression of GAB2 (but not “Empty Vector” or PAK1) induced significant expansion of hematopoietic cells from the Dnmt3aR878Hx Npm1cAmice (and to a lesser extent, from Npm1cA mice),but not Dnmt3aR878H mice or WT mice ( Figure 1A), suggesting that the mutations in Npm1 and Dnmt3a synergize to account for this phenotype. Strikingly, overexpression of GAB2 in Dnmt3aR878Hx Npm1cAbone marrow led to development of AML in 5 of 5 engrafted mice in less than 5 months (p<10 -6 compared to untransduced marrow; Figure 1B). 0 of 3 engrafted mice with “Empty Vector” (GFP only) in Dnmt3aR878Hx Npm1cAbone marrow and 0 of 3 engrafted mice with overexpression of PAK1 in Dnmt3aR878Hx Npm1cAbone marrow developed leukemia during this 5 month period. Taken together, these data suggest that AML-associated mutations in DNMT3A and NPM1 allow for the selective expansion of cells overexpressing GAB2 as an important step towards leukemic transformation. In human myeloid neoplasms, amplification of chromosome 11q (containing human GAB2) is a recurrent cytogenetic event, observed in 1.5% of cases (Papaemmanuil, NEJM, 2016), and expression of GAB2 across AML samples containing NPM1 mutations is increased ~50% compared to healthy CD34+ cells or promyelocytes. GAB2 is known to bind to GRB2 and SHP2/PTPN11 to facilitate signaling from receptor tyrosine kinases (including FLT3) to downstream pathways (including RAS/RAF/MEK/ERK, PI3K and AKT, in some contexts). These data nominate GAB2 overexpression as a factor that may be relevant for the progression of founding clones with DNMT3A and NPM1 mutations to overt AML. Additional studies to define its protein interactome in preleukemic cells, its mechanism of action, and how leukemia-causing mutations in DNMT3A and NPM1 shape the fitness landscape to select for GAB2 overexpression are underway.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal